Revolutionizing Neuroscience
Pioneering Discoveries Shaping the Future of Brain Science
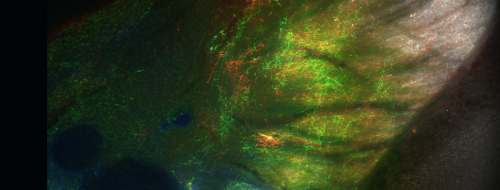
Pioneering Discoveries Shaping the Future of Brain Science

Decision-Making, Agency, and Reward Learning
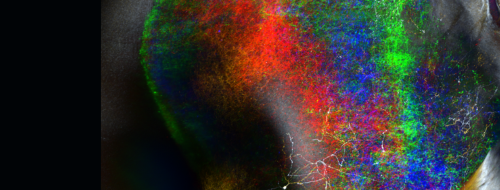
For Principles of Bio-inspired AI Architectures

Welcome! The Hamid lab opened for business in November 2021 within the Department of Neuroscience at the University of Minnesota Medical School. We are also affiliated with the Medical Discovery Team on Addiction, Neuroplasticity Research in Support of Mental Health, Graduate Program in Neuroscience, Graduate Programs in Biomedical Engineering, and Computer Science.
We are a systems and computational neuroscience research group studying brain mechanisms of Decision-making, Agency, and Reward Learning (RL).
We leverage advanced experimental and computational approaches to understand how these processes break down in debilitating psychiatric, neurological, and substance use disorders.
By reverse engineering biological algorithms for planning and behavioral control, we seek to lay the foundations for principles of bio-inspired AI architectures.
We are part of graduate training in Neuroscience, Biomedical Engineering, and Computer Science.
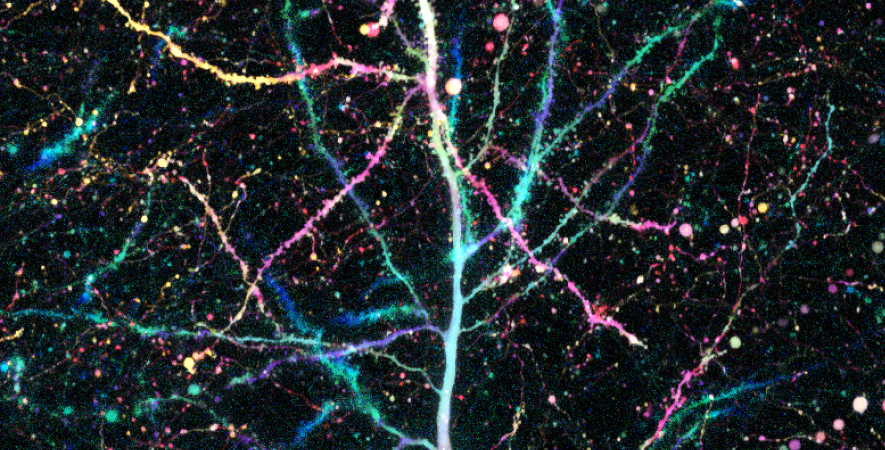
Dopamine cell firing can encode errors in reward prediction, providing a learning signal to guide future behavior. Yet dopamine is also a key modulator of motivation, invigorating current behavior. Existing theories propose that fast (phasic) dopamine fluctuations support learning, whereas much slower (tonic) dopamine changes are involved in motivation. We examined dopamine release in the nucleus accumbens across multiple time scales, using complementary microdialysis and voltammetric methods during adaptive decision-making. We found that minute-by-minute dopamine levels covaried with reward rate and motivational vigor. Second-by-second dopamine release encoded an estimate of temporally discounted future reward (a value function). Changing dopamine immediately altered willingness to work and reinforced preceding action choices by encoding temporal-difference reward prediction errors. Our results indicate that dopamine conveys a single, rapidly evolving decision variable, the available reward for investment of effort, which is employed for both learning and motivational functions.

The prairie vole is a socially monogamous rodent that is an excellent animal model for studies of the neurobiology of social attachment. Such studies have demonstrated that activation of reward circuitry during social interactions facilitates pair bond formation. Within this circuitry, μ-opioid receptors (MORs) modulate naturally rewarding behavior in an anatomically segregated manner; MORs located throughout the striatum (dorsal striatum, NAc core, and the entire NAc shell) are implicated in general motivational processes, whereas those located specifically within the dorsomedial NAc shell mediate positive hedonics (and are referred to as a "hedonic hotspot"). The purpose of the present study was to determine whether MORs within these distinct subregions differentially mediate pair bond formation. We first used receptor autoradiography to compare MOR binding densities between these regions. MOR binding was significantly higher in the NAc core and dorsomedial NAc shell compared with the ventral NAc shell. We next used partner preference testing to determine whether MORs within these subregions differentially mediate pair bonding. Blockade of MORs using 1 or 3 μg of H-d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr-NH2 within the dorsal striatum decreased mating during the cohabitation period and inhibited partner preference formation. In contrast, blockade of MORs within dorsomedial NAc shell inhibited partner preference formation without effecting mating behavior, whereas other regions were not involved. Thus, MORs within the dorsal striatum mediate partner preference formation via impairment of mating, whereas those in the dorsomedial NAc shell appear to mediate pair bond formation through the positive hedonics associated with mating.

Neurovascular coupling is a process throughwhich neuronal activity leads to local increases in blood flow in the central nervous system. In brain slices, 100% O 2 has been shown to alter neurovascular coupling, suppressing activity-dependent vasodilation. However, in vivo, hyperoxia reportedly has no effect on blood flow. Resolving these conflicting findings is important, given that hyperoxia is often used in the clinic in the treatment of both adults and neonates, and a reduction in neurovascular coupling could deprive active neurons of adequate nutrients. Here we address this issue by examining neurovascular coupling in both ex vivo and in vivo rat retina preparations. In the ex vivo retina, 100% O 2 reduced light-evoked arteriole vasodilations by 3.9-fold and increased vasoconstrictions by 2.6-fold. In vivo, however, hyperoxia had no effect on light-evoked arteriole dilations or blood velocity. Oxygen electrode measurements showed that 100% O 2 raised pO 2 in the ex vivo retina from 34 to 548 mm Hg, whereas hyperoxia has been reported to increase retinal pO 2 in vivo to only ∼53 mm Hg [Yu DY, Cringle SJ, Alder VA, Su EN (1994) Am J Physiol 267:H2498-H2507]. Replicating the hyperoxic in vivo pO 2 of 53 mm Hg in the ex vivo retina did not alter vasomotor responses, indicating that although O 2 can modulate neurovascular coupling when raised sufficiently high, the hyperoxia-induced rise in retinal pO 2 in vivo is not sufficient to produce a modulatory effect. Our findings demonstrate that hyperoxia does not alter neurovascular coupling in vivo, ensuring that active neurons receive an adequate supply of nutrients.
Our research thrives through the invaluable support and collaboration of esteemed institutions and companies. These partnerships fuel our advancements in understanding the complexities of the brain, driving innovation and excellence in neuroscience. We are grateful for their contributions and commitment to pushing the boundaries of scientific knowledge.








Our science is possible thanks to the support of our contributors. At Hamid Lab, we delve into the intricate workings of the human brain, pushing the boundaries of neuroscience research. Our groundbreaking discoveries and innovative approaches are fueled by the generous support of our funders and stakeholders, whose commitment to advancing scientific knowledge makes our work possible.





If you have any questions or want to know how you can contribute to our research, please reach us by clicking the button below.